Medicine Recall Latanoprost Timolol Eye drops
FDC Pharma are recalling a batch of Latanoprost / Timolol 50 micrograms/mL + 5mg/mL Eye Drops, Solution (PL 35638/0004) due to an out of specification result for an unknown impurity during stability testing.
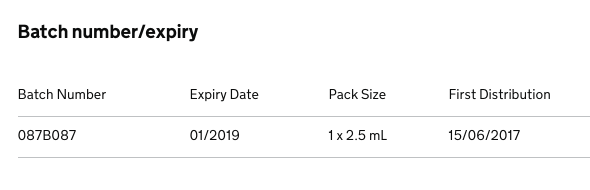
Healthcare Professionals please take the following actions:
Stop dispensing the batch listed above. Quarantine and return all remaining stock of this batch to your supplier using the supplier’s approved process.
Wholesalers
Stop distributing the batch listed above.
Quarantine and return all remaining stock of this batch to your supplier using the supplier’s approved process.
More Information on; Medicines & Healthcare products Regulatory Agency
Source MHRA
Medicine Recall Latanoprost Timolol Eye drops
Click to rate this post!
[Total: 0 Average: 0]






No Comments