Medicine Recall: Sodium Cromoglicate 2% w/v 13.5 mL Eye Drops.
Medicines and Healthcare products Regulatory Agency: released a Class 2 Medicines Recall on Sodium Cromoglicate 2% w/v 13.5 mL Eye Drops.
A Class 2 classification, specifies a recall within 48 hours. The defect could harm the patient but is not life-threatening.
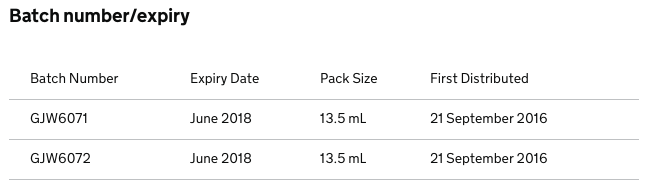
The Manufacturer FDC International Ltd is recalling the batches listed below as a precautionary measure. This is due to a precipitate observed in the bottles.
Packs with the listed batches should be quarantined and returned to warehouse.
Product information
PL number
PL15872/0010
MDR number
MDR 072-05/17
Company name
FDC International Ltd.
Product description
Sodium Cromoglicate 2% w/v 13.5 mL Eye Drops
Medicines and Healthcare products Regulatory Agency Document
Subscribe To Pharmacist Diary For Pharmacy News and Wellbeing Tips
Click to rate this post!
[Total: 0 Average: 0]






No Comments